Urinary proteomics
The project is based on capillary electrophoresis coupled to mass spectrometry (CE-MS). CE-MS analysis of urinary peptides is reproducible, robust, operator-independent, precise (including inter-laboratory precision) and stable (1). Results from a multicentre prospective study confirmed that peptide detection using CE-MS is insensitive to sample collection, handling, and storage in different centres (1). The method was found to be acceptable by the regulatory agencies European Medicines Agency (EMA) and US Food and Drug Administration (FDA). In the context of early detection of CKD, the approach has received a “Letter of Support from the FDA (2).
Capillary Electrophoresis coupled to Mass Spectrometry to assess endogenous peptides w/o digest
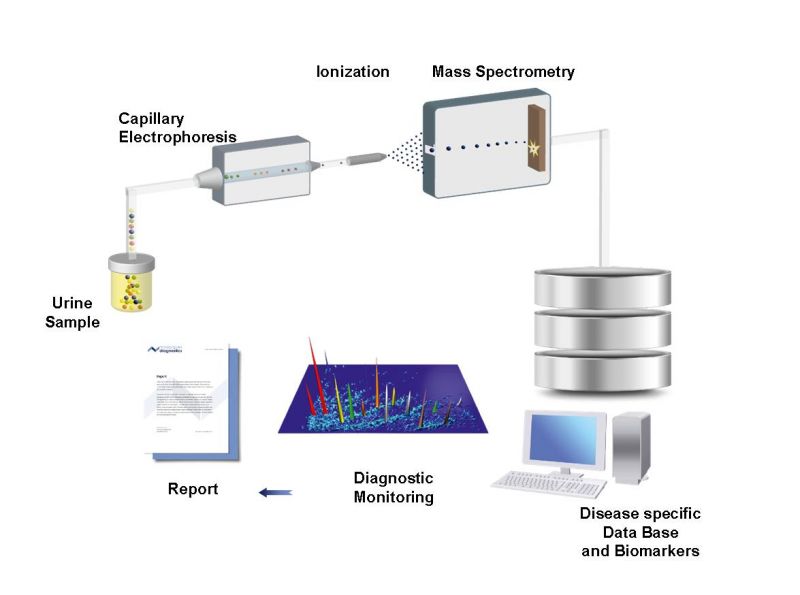
The CE-MS enable analysis of urine paptides and proteins of IgAN patients and definition of peptides that distinguish the four defined group of IgAN treated patients: supportive therapy high risk/responder, IS plus supportive therapy high risk/responder, supportive therapy low risk/non-responder and IS plus supportive therapy low risk/non-responder. The peptides associated with specific conditions will be combined in classifiers for these conditions which will be used for IgAN patient stratification.
References: 1. H. Mischak, A. Vlahou, J. P. Ioannidis, Clin. Biochem. 46, 432 (2013). 2. Biomarker Letter of Support. FDA . 14-6-2016
