Specific objectives
(A) Evaluation of baseline urine proteome of patients from previously collected samples of biopsy proven IgAN to define a proteomic classifer predicting outcome and response to IS therapy.
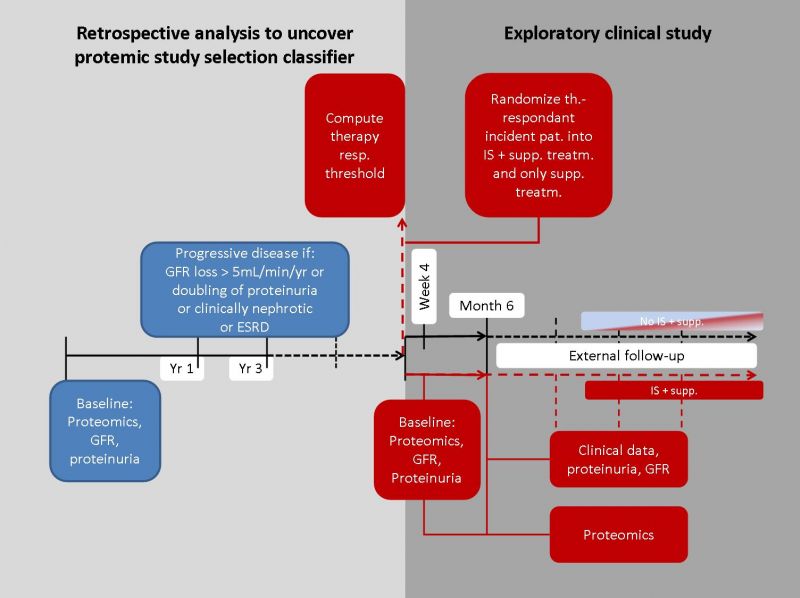
• Pre-classification of retrospective samples with known clinical outcome after at least 2 years into 4 groups: a) Progressive disease without IS; b) progressive disease with IS, c) non-progressive disease without IS; d) non-progressive disease with IS.
• Definition of “progressive disease” by glomerular filtration rate (GFR) loss exceeding an average of 5mL/min/yr for a minimum observation period of 2 years.
• Collection of baseline histological and clinical data (presence of glomerular crescent, blood pressure, magnitude of proteinuria) as alternative (non-proteomic) risk assessment of IgAN, not directly included into study design, but to compare with the proteomic classifier.
• Usage of proteomic classifier based on therapy-responding groups and generation of selection criterion to be used in a subsequent exploratory study. If therapy response cannot be predicted by urine proteome classifier, the selection criterion should be based on progression risk.
(B) Usage of this selection criterion to draft an exploratory clinical study.
• Development of a controlled (randomized) parallel-group exploratory, open-label study and implementation of this application with clinical partners recruiting incident patients. Patients joining the study will be followed until a 6-month-visit to control for proteinuria within the project frame.
• Randomization of patients fulfilling the selection criterion into only supportive treatment (ACE/ARBs) or IS (any dosage, any drug class) on top of supportive treatment by non-blinded prescription of standard approved substances depending on the personal judgement of investigating physician.
• Usage of proteinuria (PU, mg/g Crea) as a surrogate of therapy response after 6 months.
• Collection of histological and clinical data as in the retrospective study at baseline and 6 months.
• As an extension to the recent project, the partners will aim at following these patients until at least 50% of patients have reached a combined endpoint of 40% GFR loss vs. baseline or ESRD or death. The consortium will seek alternative funding and/or partners may decide to follow patients by their own resources to extend this study until these endpoints are reached.
(C) Investigate individual proteomic changes in IS and non-IS groups by a follow-up proteomic visit within exploratory clinical study.
• Collection of urine samples at early (3-4 weeks) and late (6 months) time points after IS initiation and investigation of the urine proteome, to assess the value of urinary proteome analysis in (early) detection of response to therapy.